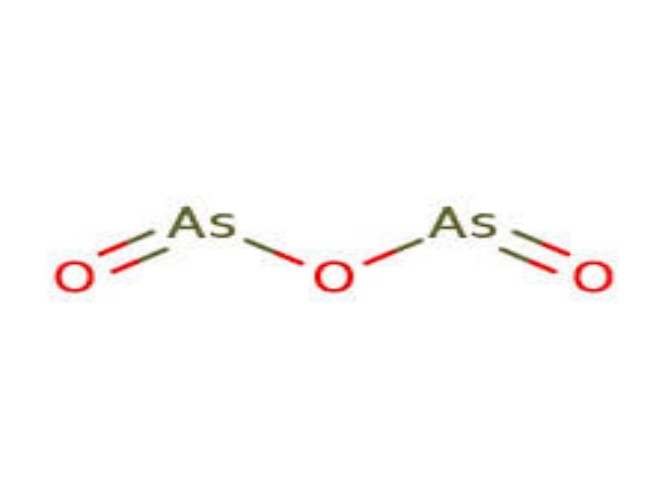
Physical Properties
- Appearance: White crystalline powder or colorless crystalline solid
- Solubility: Slightly soluble in water, more soluble in hot water
- Melting Point: Decomposes at high temperatures
- Odor: Odorless
- Toxicity: Highly toxic; lethal dose in humans is around 0.1 g
Chemical Properties
Arsenic trioxide is an amphoteric oxide that predominantly exhibits acidic properties. It readily dissolves in alkaline solutions to form arsenites. In water, it hydrolyzes to form arsenious acid (As(OH)₃), which is a weak acid.
Preparation
Arsenic trioxide is typically produced by roasting arsenic-containing minerals, such as arsenopyrite (FeAsS), in air at temperatures between 650-700°C. It can also be obtained as a by-product during the smelting of copper and lead ores that contain arsenic.
Applications
- Medical Use: Arsenic trioxide is used as a second-line treatment for acute promyelocytic leukemia (APL) due to its ability to induce apoptosis in cancer cells. It is administered intravenously under strict medical supervision.
- Industrial Use: It serves as a decolorizing agent in glass production, a preservative for wood, and a starting material for various arsenic compounds.
- Historical Uses: Arsenic trioxide has been used historically as a poison, in rat poison, and as a pigment in paints.
Safety and Health Hazards
- Toxicity: Arsenic trioxide is extremely toxic and can cause acute gastrointestinal and central nervous system symptoms. Chronic exposure may lead to respiratory issues, skin lesions, and an increased risk of cancer.
- Handling: It should be stored in tightly closed containers in a cool, dry, and well-ventilated area. Protective equipment is essential when handling this compound.
Environmental Impact
Arsenic trioxide is a confirmed carcinogen and poses significant environmental risks. It requires careful disposal to prevent contamination of water sources and soil.
Summary
Arsenic trioxide is a versatile but highly toxic compound with applications in both medicine and industry. Its use is tightly regulated due to its potential for harm, and it must be handled with extreme caution.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping