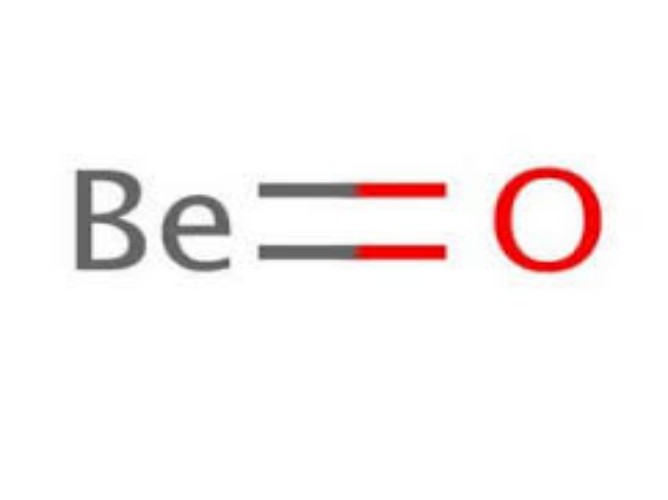Physical Properties
- Molar Mass: 25.01 g/mol
- Density: 3.01 g/cm³
- Melting Point: 2507°C
- Boiling Point: 3600°C
- Appearance: White crystalline solid
- Solubility: Insoluble in water, soluble in acids and bases.
- Crystal Structure: Hexagonal.
Preparation
Beryllium oxide can be prepared by the thermal decomposition of beryllium nitrate or beryllium carbonate. It can also be produced by reacting beryllium metal with oxygen at high temperatures.
Applications
- Electronics: Used as an insulating material due to its high thermal conductivity and electrical resistivity.
- Ceramics: Employed in the production of advanced ceramic materials for its excellent mechanical properties and thermal stability.
- Nuclear Industry: Utilized in nuclear reactors as a neutron reflector and moderator due to its low neutron absorption cross-section.
- Optics: Used in the manufacturing of high-performance optical materials.
Safety and Health Considerations
Beryllium oxide is highly toxic, and inhalation or ingestion can cause serious health issues, including berylliosis, a chronic lung disease. It is classified as a carcinogen, and strict safety measures are required to prevent exposure.
Environmental Impact
Due to its toxicity, beryllium oxide requires careful handling and disposal to prevent environmental contamination. It is important to follow regulatory guidelines for its use and disposal.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping