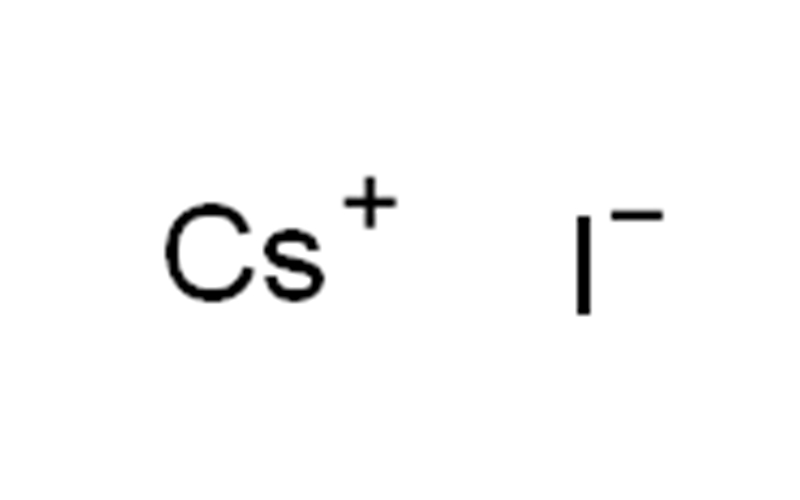Cesium iodide Chemical Properties
| Melting point | 626 °C (lit.) |
| Boiling point | 1280 °C |
| density | 4.51 g/mL at 25 °C (lit.) |
| refractive index | 1.7876 |
| Fp | 1280°C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | soluble in ethanol, methanol, acetone |
| form | beads |
| Specific Gravity | 4.51 |
| color | White |
| PH | pH(50g/l, 25℃) : 5.0~9.0 |
| Water Solubility | 74 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Boiling Point | 1280 °C |
| Exposure limits | ACGIH: TWA 0.01 ppm |
| Dielectric constant | 5.6(Ambient) |
| Stability: | Stable. Deliquescent. |
Safety Information
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 2 |
| RTECS | FL0350000 |
| TSCA | Yes |
| HS Code | 28276000 |
| Toxicity | LD50 i.p. in rats: 1.4 g/kg (Cochran) |
Cesium iodide Usage And Synthesis
| Description | Cesium iodide is a soft material and extremely hygroscopic. Cesium iodide scintillators have been used extensively for indirect x-ray imaging detectors, such as for CT examinations. Cesium iodide is also used to construct electromagnetic shower detector for the CLEO II experiment, which investigates electron-positron annihilation at center-of-mass energies near 10 GeV. Cesium iodide is useful for beam splitters for Far IR spectrophotometers and IR transmission windows. |
| References | [1] W. Zhao, G. Ristic, J. A. Rowlands, X-ray imaging performance of structured cesium iodide scintillators, Medical Physics, 2004, vol. 31, 2594-605 [2] E. Blucher, B. Gittelmann, B. K. Heltsley, J. Kandaswamy, R. Kowalewski, Y.Kubota, N. Mistry and S. Stone, Tests of cesium iodide crystals for an electromagnetic calorimeter, Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 1986, vol. 249, 201-227 [3] M. Völk, O. W. Hamer, S. Feuerbach, M. Strotzer, Dose reduction in skeletal and chest radiography using a large-area flat-panel detector based on amorphous silicon and thallium-doped cesium iodide: technical background, basic image quality parameters, and review of the literature, European Radiology, 2004, vol. 14, 827-834 |
| Chemical Properties | white crystalline powder |
| Uses | Prisms for infrared spectroscopy; in x-ray fluorescent screens, scintillation counters. |
| Uses | Cesium iodide (CsI) is a kind of ionic compound that has been widely used as scintillators in many areas such as X-ray image intensifier tubes, photocathodes, and display devices. Cesium iodide is used in scintillation counters(Geiger counters) to measure levels of external radiation. It is also useful as a “getter” to remove air molecules remaining in vacuum tubes. Cesium Iodide is a material with high γ-ray stopping power due to its relative high density and atomic number. For scintillation counting, it is used either in its un-doped form or doped with sodium or thallium. CsI is resistant to thermal and mechanical shock. The physical characteristics of CsI are independent of the activator used. Compared to NaI(Tl), it is relatively soft and plastic, and does not cleave. Because it has no cleavage plane, it is quite rugged-which makes it well-suited for well logging, space research or other applications where severe shock conditions are encountered. |
| Uses | Cesium iodide is used in FT-IR (Fourier Transform Infrared Spectrometers) as a beamsplitter. Cesium Iodide exhibits high gamma-ray stopping power. Useful as input phosphor in fluoroscopy equipment, and efficient photocathode for extreme ultraviolet wavelengths. Fast and dense scintillating material – useful in electromagnetic calorimetry in particle physics. |
| Production Methods | A single crystal can be grown from a melt solution by both the Kyropoulus method and the Stockbarger method. |
| General Description | Cesium iodide is a simple ionic salt. It has the capacity to undergo second order transformation from cubic B2 structure to body-centered tetragonal structure at low pressures. It is mostly used as input screens of X-ray image intensifiers. |
| Flammability and Explosibility | Non flammable |
| Safety Profile | Moderately toxic by ingestion and intraperitoneal routes. See also CESIUM and IODIDES. When heated to decomposition, it emits toxic fumes of I-. |
| Solubility in water | Cesium iodide is soluble in water, with a solubility of 44/100 g H2O (0℃ and 160/100 g H2O (61℃). It is soluble in alcohol. One should handle it carefully, because it is deliquescent. |
| Purification Methods | Crystallise it from warm water (1mL/g) by cooling to -5o. |
| Structure and conformation | The space lattice of CsI belongs to the cubic system, and its cesium chloride structure has a lattice constant of a=0.8529 nm. |
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping