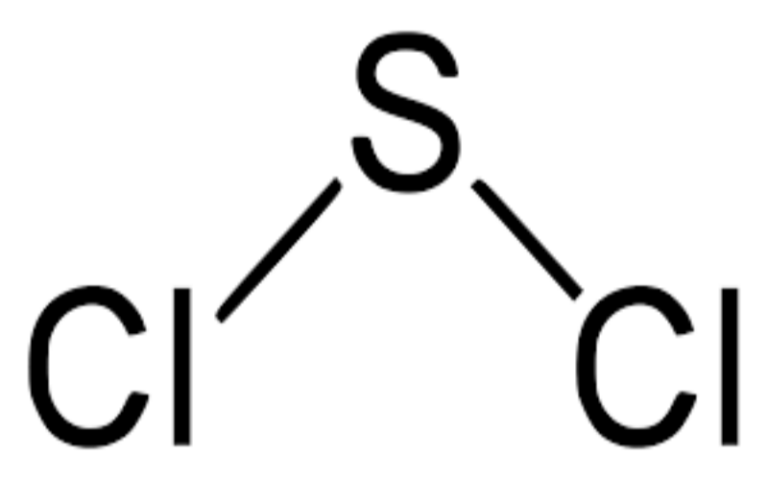
Physical Properties
- Appearance: Colorless gas with a pungent odor.
- Density: 1.774 g/L (estimate).
- Melting Point: -103°C.
- Boiling Point: -13.1°C.
- Solubility: Soluble in water, but reacts violently to produce hydrofluoric acid and toxic chloride gas.
Chemical Properties
- Reactivity:
- Strong oxidizing agent and fluorinating agent.
- Reacts violently with water, ice, and many metals.
- Decomposes to release toxic fumes of chlorine and fluorine when heated.
- Decomposition: Releases highly toxic gases (e.g., HF, Cl₂) upon contact with moisture or heat.
Uses
- Rocket Propellants: Used as an oxidizer in rocket fuels.
- Fluorinating Agent: Used in chemical synthesis to introduce fluorine atoms.
- Semiconductor Industry: Used for etching silicon wafers.
- Nuclear Fuel Processing: Used in the production of uranium hexafluoride.
Safety and Hazards
- Toxicity: Highly toxic; inhalation can cause severe respiratory issues and burns.
- Corrosivity: Corrosive to skin and metals; causes severe burns upon contact.
- Storage: Store in airtight containers, away from water and moisture.
- Handling: Requires specialized equipment and protective gear due to its highly reactive nature.
- Fire Hazard: Supports combustion and can ignite non-flammable materials like sand or asbestos.
Environmental Impact
- Toxic Gases: Reacts with water to produce toxic gases like hydrofluoric acid (HF) and chlorine gas (Cl₂).
- Acid Rain: Can contribute to acid rain if released into the atmosphere.
- Soil and Water Contamination: Potential to contaminate soil and water sources.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping