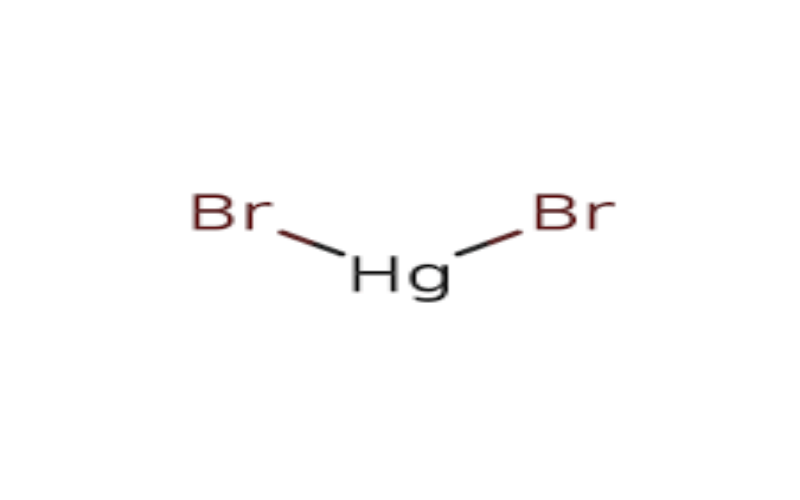
Physical Properties
Boiling Point: 322°C.- Density: 6.1 g/cm³.
- Solubility: Slightly soluble in water (6.1 mg/mL at 25°C), more soluble in ethanol.
Chemical Properties
- Stability: Stable but sensitive to light; darkens upon exposure.
- Reactivity: Reacts with reducing agents to form elemental mercury. It can also react with sodium azide to form shock-sensitive compounds.
- Formation: Can be synthesized by reacting metallic mercury with bromine.
Uses
- Laboratory Reagent: Used in the Koenigs–Knorr reaction and as a promoter in glycosylation reactions.
- Other Applications: Used in some analytical procedures and as a precursor for other mercury compounds.
Safety Information
- Toxicity: Highly toxic; inhalation, ingestion, and skin contact can cause severe health effects.
- Health Hazards: Causes severe irritation to skin, eyes, and mucous membranes. Contact with molten material may cause burns.
- First Aid:
- Skin Contact: Rinse with plenty of water.
- Eye Contact: Rinse with water for at least 15 minutes.
- Inhalation: Move to fresh air.
- Ingestion: Do not induce vomiting; seek medical attention.
- Storage: Store in a cool, dry place, away from light and reducing agents.
Environmental Information
- Hazardous Decomposition Products: May release toxic fumes of hydrogen bromide and mercury upon heating or combustion.
Transport Information
- UN Number: UN1634.
- Hazard Class: Poison.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping