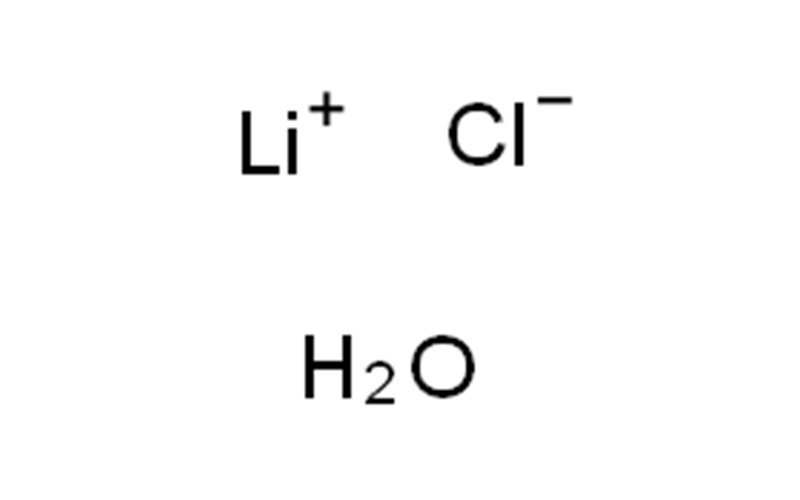Lithium chloride monohydrate Chemical Properties
| Melting point | >98°C -H₂O |
| Boiling point | 1382℃ |
| density | 1.78 |
| solubility | very soluble in H2O |
| form | Granules |
| color | White |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| Merck | 14,5528 |
Basic Information
- Chemical properties: White crystalline solid with strong salt odor, highly hygroscopic. Dissolve in water, alcohol, acetone, pentanol, pyridine, and nitrobenzene. pH <7。
- Usage: Chemical synthesis; Materials Science.
- Storage: Ordinary temperature
- Hazard statement: Harmful and irritating.
- Preventive statement: Avoid inhalation. Wear protective clothing/gloves/goggles/masks. Extinguishing: fog water, alcohol resistant foam, sand, dry powder, carbon dioxide. Avoid taboo items.
Lithium chloride monohydrate Usage And Synthesis
| Chemical Properties | white crystal(s); prepared by reacting HCl with LiOH or Li2CO3 and crystallizing below 95°C; solution is used for deicing, in fire extinguishers, catalysts and for dehumidifying [FMC93] [KIR81] [STR93] |
| Uses | Lithium chloride monohydrate is used as a precursor in the production of lithium metal and other lithium compounds. It acts as a welding agent for aluminum, salt bath for heat -treatment by low temperature and soldering techniques. It is also used as a tracer for chemical products viz. denaturation of wine. For absorbers, it is an absorption and disinfection reagent as well as desiccant for air conditioner. |
Packing and shipping