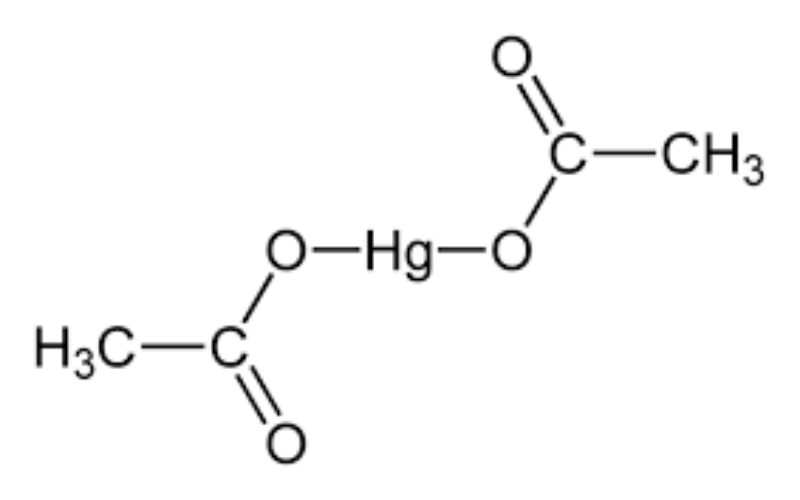
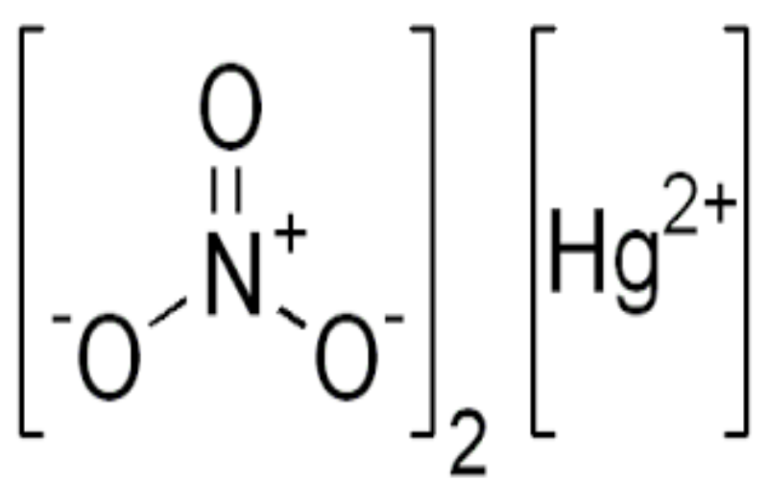
Physical Properties
- Appearance: White crystalline powder or pale yellow powder with an acetic odor.
- Melting Point: 178-180°C.
- Density: 3.29 g/cm³.
- Solubility: Soluble in water, acetic acid, and dichloromethane; slightly soluble in ethanol; insoluble in benzene and hexane.
- Stability: Stable but decomposes slowly in water to form mercury(II) oxide (HgO) and acetic acid.
- Sensitivity: Light-sensitive.
Chemical Properties
- Reactivity: Reacts with strong oxidizing agents and bases. Decomposes upon exposure to light.
- Formation: Prepared by reacting acetic acid with mercuric oxide.
Uses
- Organic Synthesis: Used as a catalyst in organic reactions, such as hydromercuriation of alkenes.
- Analytical Reagent: Used in chemical analysis.
- Medicinal Chemistry: Used in the synthesis of pharmaceuticals.
- Gas Analysis: Used to absorb ethylene in gas analysis.
Safety Information
- Toxicity: Highly toxic; acute oral LD₅₀ in rats: 40,900 μg/kg; in mice: 23,900 μg/kg.
- Health Hazards: Causes severe irritation to skin and eyes; inhalation and ingestion are extremely hazardous.
- Risk Phrases:
- R26/27/28: Very toxic by inhalation, in contact with skin, and if swallowed.
- R33: Danger of cumulative effects.
- R50/53: Very toxic to aquatic organisms, may cause long-term adverse effects.
- Safety Phrases:
- S13: Keep away from food, drink, and animal feeding stuffs.
- S28: After contact with skin, wash immediately with plenty of soap and water.
- S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible).
- S60: This material and its container must be disposed of as hazardous waste.
- S61: Avoid release to the environment. Refer to special instructions/Safety data sheets.
Handling and Storage
- Store in a cool, dry place, away from light and strong oxidizing agents.
- Use appropriate personal protective equipment (PPE) to avoid contact with skin and eyes.
Environmental Information
- Extremely hazardous to aquatic life; avoid release into the environment.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping