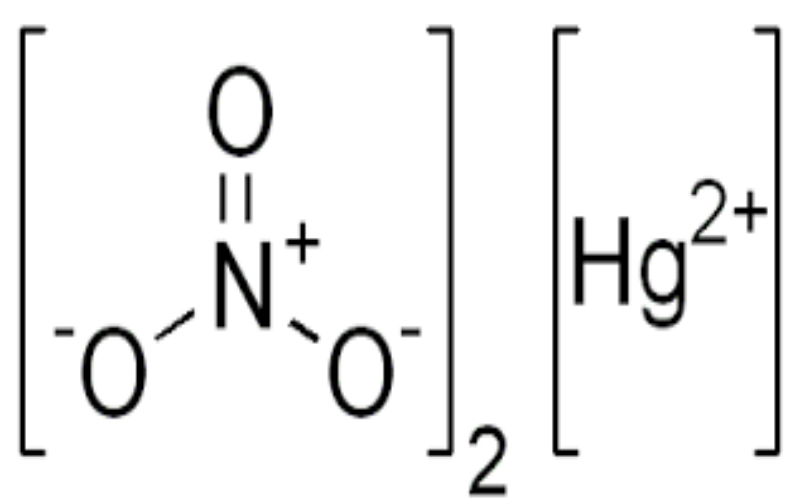
Physical Properties
- Appearance: White to yellowish crystalline solid.
- Density: 4.3 g/cm³.
- Melting Point: 79°C.
- Boiling Point: Decomposes at 180°C.
- Solubility: Soluble in water and nitric acid; slightly soluble in ethanol.
- Sensitivity: Hygroscopic and light-sensitive.
Chemical Properties
- Stability: Stable but decomposes upon heating to form mercury(II) oxide, nitrogen dioxide, and oxygen.
- Reactivity:
- Reacts violently with organic materials, powdered metals, and reducing agents.
- Forms explosive mixtures with phosphorus and other reducing agents.
- Decomposes in water to form basic salts.
Uses
- Organic Synthesis: Used as a nitrating agent and catalyst.
- Analytical Reagent: Used in chemical analysis.
- Medicinal Chemistry: Used in the preparation of other mercury compounds.
- Explosives: Used in the manufacture of mercury fulminate.
Safety Information
- Toxicity: Highly toxic by ingestion and inhalation.
- Health Hazards: Causes severe irritation to skin and eyes; inhalation may lead to respiratory distress.
- First Aid:
- Skin Contact: Rinse thoroughly with soap and water.
- Eye Contact: Rinse with water for at least 15 minutes.
- Inhalation: Move to fresh air and seek medical attention.
- Ingestion: Do not induce vomiting; seek medical attention.
- Storage: Store in a cool, dry place, away from light and organic materials.
Environmental Information
- Hazardous Decomposition Products: Mercury(II) oxide, nitrogen oxides.
- Ecotoxicity: Very toxic to aquatic life.
Transport Information
- UN Number: UN1625.
- Hazard Class: 6.1 (Poisonous material).
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping