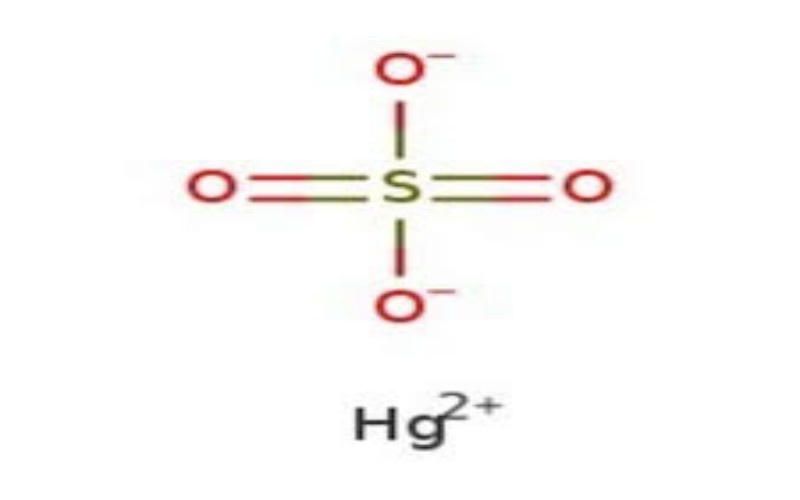
Physical Properties
- Appearance: White crystalline solid.
- Density: 6.47 g/cm³.
- Melting Point: Decomposes at 615.95°C.
- Solubility:
- Decomposes in water to form a yellow insoluble basic sulfate and sulfuric acid.
- Soluble in hot dilute sulfuric acid, concentrated sodium chloride solution, and hydrochloric acid.
- Insoluble in acetone and ammonia.
Chemical Properties
- Stability: Unstable; decomposes upon heating to form mercury(II) oxide and sulfur dioxide.
- Reactivity:
- Reacts with strong acids and reducing agents.
- Sensitive to light and heat.
Uses
- Catalyst: Used in organic reactions, such as the conversion of acetylene to acetaldehyde.
- Electrolyte: Used in primary batteries.
- Reagent: Used in chemical analysis and as a reagent for the determination of barbiturates and cysteine.
- Extraction: Used in the extraction of gold and silver from roasted pyrites.
Safety Information
- Toxicity: Highly toxic; acute oral LD₅₀ in rats: 57 mg/kg.
- Health Hazards: Causes severe irritation to skin and eyes; inhalation may lead to respiratory distress.
- First Aid:
- Skin Contact: Rinse with plenty of water.
- Eye Contact: Rinse with water for at least 15 minutes.
- Inhalation: Move to fresh air and seek medical attention.
- Ingestion: Drink plenty of water and seek medical attention.
- Storage: Store in a cool, dry place, away from strong acids and reducing agents.
Environmental Information
- Ecotoxicity: Very toxic to aquatic life; may cause long-term adverse effects in the aquatic environment.
- Hazardous Decomposition Products: Mercury(II) oxide, sulfur oxides.
Transport Information
- UN Number: UN1624.
- Hazard Class: 6.1 (Poisonous material).
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping