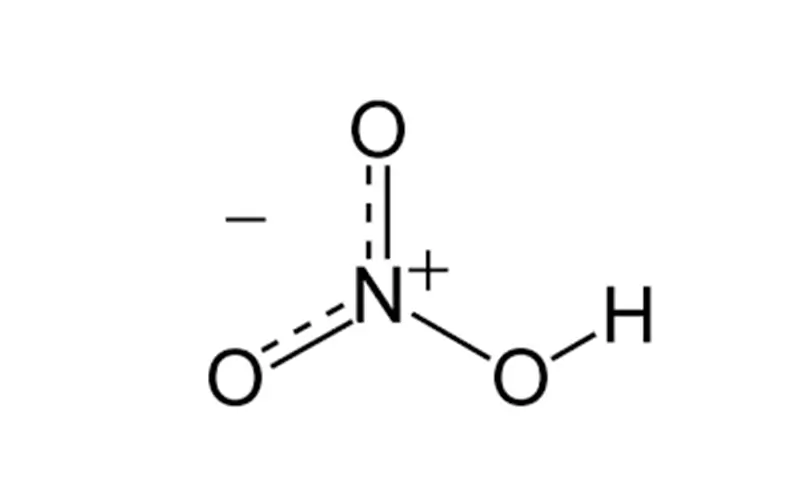
Nitric acid is a strong acid with a wide range of industrial applications. Here are some of the main uses:
- 1.Fertilizer Production: Nitric acid is an important raw material for the production of nitrogen fertilizers, such as ammonium nitrate and potassium nitrate.
- 2.Explosive Manufacturing: Nitric acid is a key component in the manufacture of certain explosives, such as nitroglycerin.
- 3.Metal Processing: Nitric acid is used for pickling metals to remove oxides and impurities from the metal surface.
- 4.Electroplating and Metal Surface Treatment: Nitric acid is used in the pre-treatment and post-treatment of electroplating, as well as for passivation treatment of metals.
- 5.Chemical Synthesis: Nitric acid is used in the synthesis of various organic compounds, such as dyes, pharmaceuticals, and plastics.
- 6.Acid Cleaning: In the oil and gas industry, nitric acid is used to remove corrosion products from pipelines and equipment.
- 7.Cleaners and Rust Removers: The strong acidity of nitric acid makes it an effective cleaner and rust remover.
- 8.Analytical Chemistry: In laboratories, nitric acid is used to analyze samples to determine the content of metals and other elements.
PHYSICAL & CHEMICAL INFORMATION
Physical State; Appearance
COLOURLESS-TO-YELLOW LIQUID WITH PUNGENT ODOUR.
Physical dangers
No data.
Chemical dangers
Decomposes on warming. This produces toxic and irritating fumes and gases including nitrogen oxides. The substance is a strong oxidant. It reacts violently with combustible and reducing materials, such as turpentine, charcoal and alcohol. The substance is a strong acid. It reacts violently with bases and is corrosive to metals. This produces flammable/explosive gas (hydrogen – see ICSC 0001). Reacts violently with organic compounds.
EXPOSURE & HEALTH EFFECTS
| Routes of exposure Serious local effects by all routes of exposure. Effects of short-term exposure The substance is corrosive to the eyes, skin and respiratory tract. Corrosive on ingestion. Inhalation may cause asthma-like reactions (RADS). Exposure could cause asphyxiation due to swelling in the throat. Inhalation of high concentrations may cause pneumonitis and lung oedema. See Notes. | Inhalation risk A harmful contamination of the air can be reached very quickly on evaporation of this substance at 20°C. Effects of long-term or repeated exposure Repeated or prolonged inhalation may cause effects on the teeth. This may result in tooth erosion. The substance may have effects on the upper respiratory tract and lungs. This may result in chronic inflammation of the respiratory tract and reduced lung function . Mists of this strong inorganic acid are carcinogenic to humans. Notes. |
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping