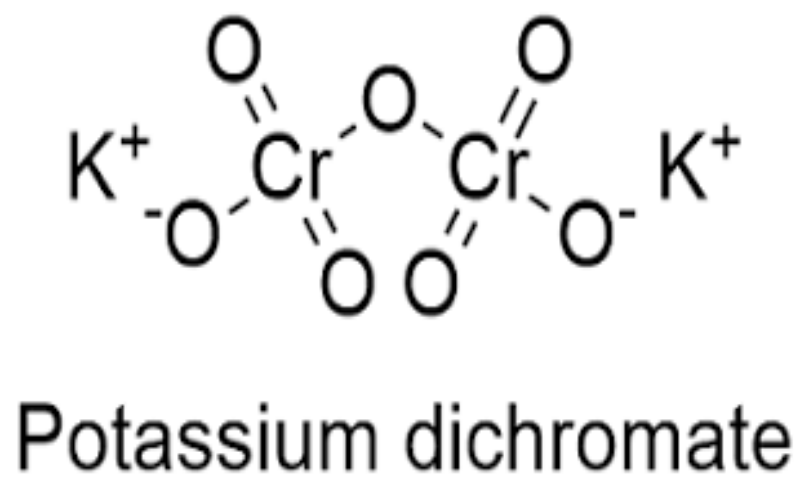
Physical Properties
- Appearance: Bright red-orange crystalline solid.
- Odor: Odorless.
- Density: 2.676 g/cm³.
- Melting Point: 398°C.
- Boiling Point: 500°C (decomposes).
- Solubility in Water:
- 4.9 g/100 mL at 0°C.
- 13 g/100 mL at 20°C.
- 102 g/100 mL at 100°C.
- Solubility in Other Solvents: Insoluble in alcohol and acetone.
Chemical Properties
- Oxidizing Agent: Potassium dichromate is a strong oxidizing agent used in various chemical reactions.
- Color Change:
- In acidic conditions, it appears orange-red (Cr₂O₇²⁻).
- In alkaline conditions, it turns yellow (CrO₄²⁻).
- Thermal Decomposition:
- Decomposes at high temperatures to form potassium chromate, chromium(III) oxide, and oxygen.
- Reaction: 4K₂Cr₂O₇ → 4K₂CrO₄ + 2Cr₂O₃ + 3O₂.
Uses
- Analytical Chemistry: Used as a reagent for detecting ferrous salts, iodides, and sulfides.
- Leather Tanning: Used as a precursor for potassium chrome alum.
- Photography: Used in photographic screen printing.
- Glass Cleaning: Historically used as a cleaning agent for glassware (though discontinued due to safety concerns).
- Medical Uses: Used externally as an antiseptic, caustic, and astringent.
Safety Information
- Health Hazards:
- Potassium dichromate is a known human carcinogen, particularly affecting the respiratory tract.
- Causes severe irritation to skin and eyes; ingestion can lead to chemical burns.
- Storage: Store in a cool, dry place, away from reducing agents and organic materials.
- First Aid:
- Skin Contact: Rinse with plenty of water.
- Eye Contact: Rinse with water for at least 15 minutes.
- Inhalation: Move to fresh air and seek medical attention.
- Ingestion: Drink water and seek medical attention.
Environmental Information
- Ecotoxicity: Highly toxic to aquatic life and may have long-term adverse effects.
- Decomposition Products: Chromium(III) oxide, oxygen.
Transport Information
- UN Number: 3288.
- Hazard Class: 6.1 (Toxic substances).
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping