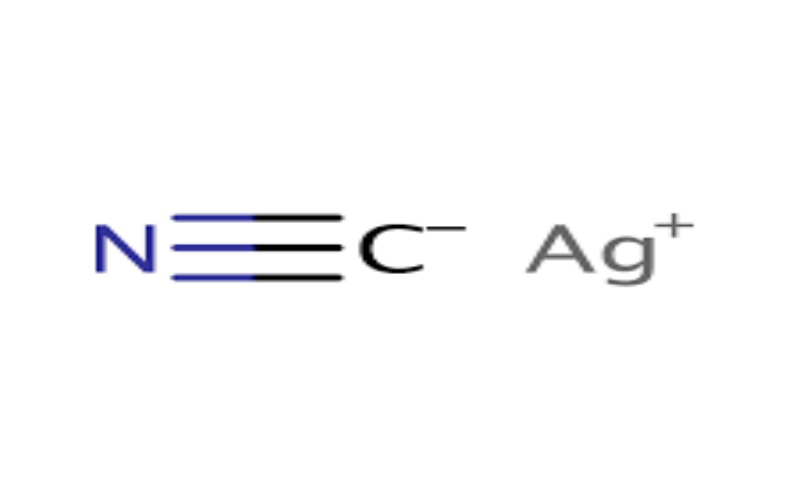
Physical Properties
- Appearance: White to light gray powder.
- Odor: Odorless.
- Density: 3.95 g/cm³.
- Melting Point: 300°C.
- Boiling Point: 25.7°C at 760 mmHg.
- Solubility:
- Insoluble in water and ethanol.
- Soluble in ammonia, hot concentrated nitric acid, and thiosulfate solutions.
Chemical Properties
- Stability: Stable in dry air; decomposes above 320°C.
- Reactivity:
- Reacts with acids to release toxic hydrogen cyanide gas.
- Forms complexes with alkali metal cyanides.
- Photoreactivity: Darkens upon exposure to light.
Synthesis
- Metal Silver Method: Silver is dissolved in nitric acid, diluted with water, and reacted with sodium cyanide to form a precipitate of silver cyanide.
- Reaction: Ag + 2HNO₃ → AgNO₃ + H₂O + NO₂↑
- AgNO₃ + NaCN → AgCN↓ + NaNO₃.
- Double Decomposition Method: Silver nitrate reacts with potassium cyanide to form silver cyanide.
- Reaction: AgNO₃ + KCN → AgCN↓ + KNO₃.
Uses
- Medicine: Used in pharmaceuticals.
- Electroplating: Used for silver plating and coating bearings in aircraft engines.
- Protective Coatings: Used as a lining for pressurized heat exchangers.
- Catalyst: Used in organic synthesis and as a catalyst for the synthesis of heterocyclic compounds.
Safety Information
- Toxicity: Highly toxic; ingestion, inhalation, and skin contact can be fatal.
- Health Hazards: Causes severe irritation to skin and eyes; exposure to light darkens the compound.
- First Aid:
- Skin Contact: Rinse with soap and water.
- Eye Contact: Rinse with water for at least 15 minutes.
- Ingestion: Drink water and seek medical attention.
- Storage: Store in a cool, dry place, away from light and acids.
- Transportation: UN Number 1684; classified as a toxic substance.
Environmental Information
- Ecotoxicity: Highly toxic to aquatic life and may cause long-term adverse effects.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping