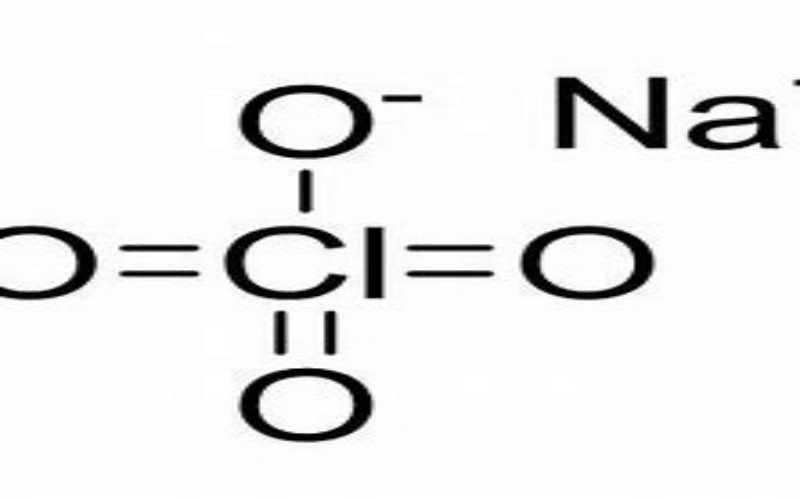
Physical Properties
- Appearance: White crystalline powder or prismatic crystals, hygroscopic.
- Melting Point: 482°C (decomposes).
- Density: 2.53 g/cm³ (relative density).
- Solubility: Soluble in water (209 g/100 mL at 15°C), ethanol, and acetone; insoluble in ether.
Chemical Properties
- Oxidizing Agent: Strong oxidizer with potential to explode when mixed with organic materials, reducing agents, or concentrated sulfuric acid.
- Stability: Unstable at high temperatures; decomposes upon heating to 482°C.
- Reactivity: Reacts violently with combustible materials, potentially causing fires or explosions.
Uses
- Industrial Applications: Used in the manufacture of other perchlorate salts (e.g., potassium perchlorate) and explosives.
- Analytical Reagent: Used as an oxidizing agent in chemical analysis.
Safety and Hazards
- Health Hazards: Causes severe irritation to skin and mucous membranes. Ingestion is harmful.
- Environmental Hazards: Limited data available, but it is considered harmful to aquatic life.
- Storage and Handling: Store in a cool, dry place away from combustible materials, organic substances, and reducing agents. Use protective equipment when handling.
Emergency Measures
- Leakage: Isolate the contaminated area, avoid contact with organic materials, and collect the material in a dry container for disposal.
- First Aid: In case of skin contact, rinse thoroughly with water. If ingested, seek medical attention.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping