properties
White crystal or powder, belonging to the orthorhombic crystal system, with a density of 2.7 g/cm3 and a melting point of 884 ℃; Soluble in water, soluble in glycerol, insoluble in ethanol. The aqueous solution is neutral. Exposed to air, it is easy to absorb moisture and form hydrated sodium sulfate. There are two types of crystalline water compounds, one is sodium sulfate heptahydrate Na2SO4 · 7H2O, which is a white hexagonal or tetragonal crystal and loses water at 24.4 ℃. Another type is sodium sulfate decahydrate Na2SO4 · 10H2O, which is a colorless monoclinic crystal with a density of 1.46 g/cm3. It is easily soluble in water and loses its crystalline water to become anhydrous sodium sulfate at 100 ℃. It is prone to weathering in dry air and becomes an anhydrous white powder.
Sodium sulfate is chemically stable, insoluble in strong acids and bases, hygroscopic, and can undergo metathesis reactions with some salts to form precipitates:
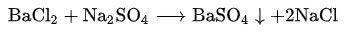
Usage
Sodium sulfate is an important chemical raw material, which is the main raw material for the production of chemical products such as sodium sulfide and sodium silicate. It can also be used as a filler for synthetic detergents, a cooking agent for the production of sulfate pulp in the paper industry, and a diuretic, laxative, and detoxifying agent for barium salts in medicine.
precautions
Store in a cool and ventilated warehouse, away from sources of fire and heat, and separate from acids for storage and transportation.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping