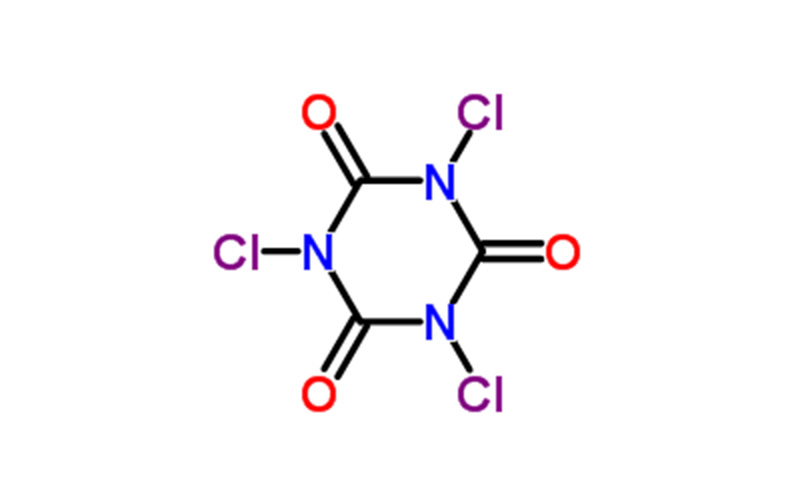
Trichloroisocyanuric acid (TCCA) is a highly efficient, broad-spectrum, and safe disinfectant with strong oxidizing and chlorinating properties. Its main uses include:
- 1.Water Treatment Disinfection: Widely used for the sterilization and disinfection of drinking water, swimming pool water, industrial circulating water, and sewage.
- 2.Medical Field: Used for sterilization and disinfection in hospitals and for tableware.
- 3.Aquaculture Applications: As a broad-spectrum antibacterial agent for marine disinfection, used in the treatment and prevention of aquatic animal diseases.
- 4.Food Processing: Used for washing fruits, vegetables, and meat, as well as for disinfecting food production lines.
- 5.Textile Industry: Used as a washing and bleaching agent for cotton, linen, and synthetic fabric.
- 6.Wool Anti-shrink: Used as a wool anti-shrink agent in the wool textile industry.
- 7.Rubber Industry: Used as a chlorinating agent in rubber industry production.
- 8.Organic Synthesis: Used as a raw material in the organic synthesis industry to synthesize various organic compounds, such as tri(2-hydroxyethyl) isocyanurate.
- 9.Industrial Oxidizing Agent: Its oxidation-reduction electrode potential is equivalent to that of hypochlorite, and it can be used as a high-quality oxidizing agent to replace hypochlorite.
- 10.Agricultural Applications: Used for rice seed treatment and fruit preservation.
- 11.Daily Chemical Industry: Used for decolorization in daily chemicals, wood mold prevention, papermaking, etc.
- 12.Industrial Cleaning: Used as a cleaning agent in industrial cleaning.
PHYSICAL & CHEMICAL INFORMATION
Physical State; Appearance
WHITE CRYSTALLINE POWDER WITH PUNGENT ODOUR.
Physical dangers
No data.
Chemical dangers
Decomposes on heating. This produces toxic fumes. May explode on heating. The substance is a strong oxidant. It reacts with combustible and reducing materials. Reacts violently with ammonia, ammonium salts and amines and sodium carbonate (soda ash) . This generates fire and explosion hazard. Reacts with strong acids. This produces toxic gas (chlorine – see ICSC 0126).
- Formula: C3Cl3N3O3
- Molecular mass: 232.4
- Decomposes at >225°C
- Density: 2.07 g/cm³
- Solubility in water, g/100ml at 25°C: 1.2
- Vapour pressure: negligible
- Octanol/water partition coefficient as log Pow: 0.26
EXPOSURE & HEALTH EFFECTS
| Routes of exposure The substance can be absorbed into the body in hazardous amounts by inhalation and by ingestion. Effects of short-term exposure The substance is severely irritating to the eyes and respiratory tract. The substance is mildly irritating to the skin. Corrosive on ingestion. Inhalation of dust may cause lung oedema. See Notes. | Inhalation risk A harmful concentration of airborne particles can be reached quickly when dispersed. Effects of long-term or repeated exposure |
SPILLAGE DISPOSAL: Personal protection: particulate filter respirator adapted to the airborne concentration of the substance. Do NOT let this chemical enter the environment. Sweep spilled substance into covered dry, sealable containers. Carefully collect remainder. Then store and dispose of according to local regulations.
STORAGE: Dry. Well closed. Separated from amines, combustible substances and reducing agents. See Chemical Dangers. Store in an area without drain or sewer access. Provision to contain effluent from fire extinguishing.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping