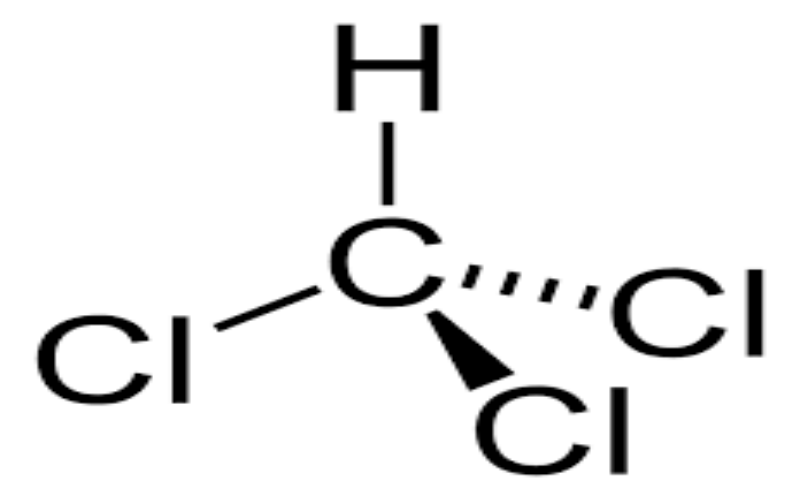
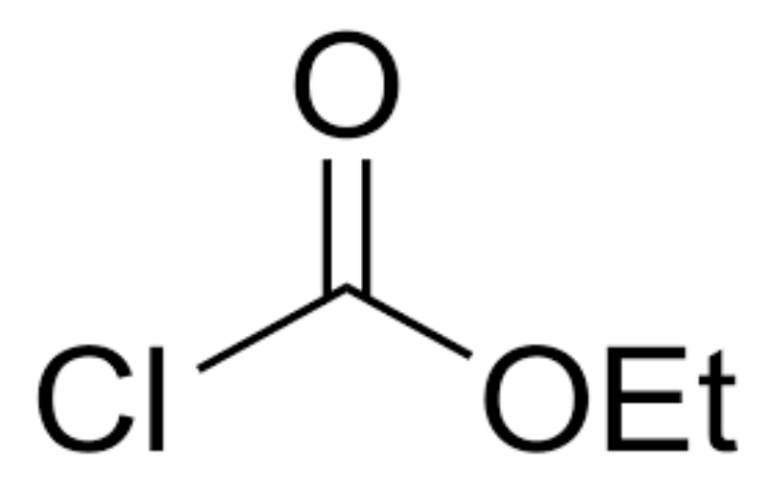
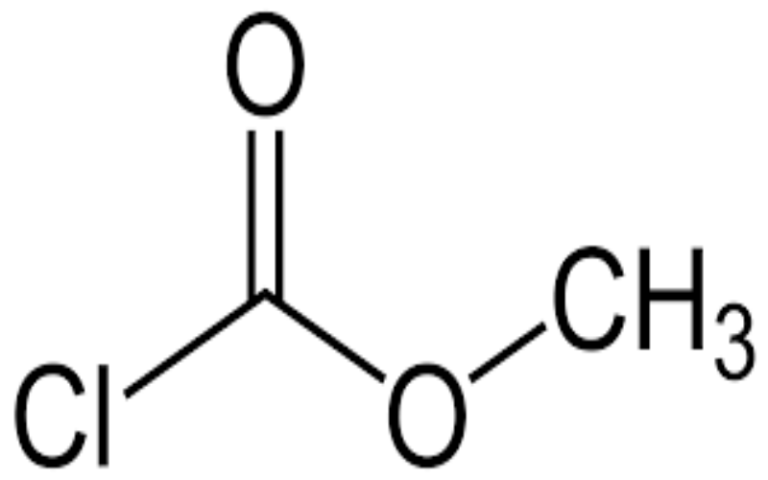
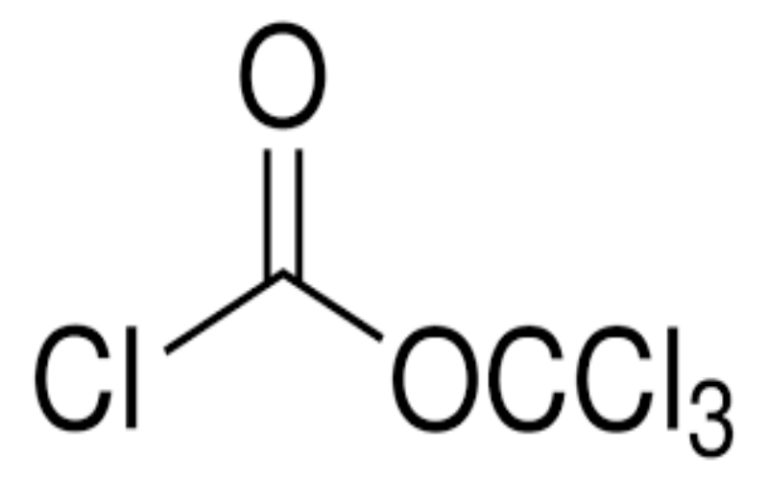
Physical Properties
- Appearance: Colorless, transparent liquid with a sweet odor.
- Melting Point: -63.5°C.
- Boiling Point: 61.12°C.
- Density: 1.48 g/cm³.
- Solubility: Insoluble in water, soluble in ethanol, ether, and benzene.
- Refractive Index: High.
Chemical Properties
- Stability: Stable under normal conditions but sensitive to light. Decomposes in the presence of light and air to form phosgene (a highly toxic gas) and hydrogen chloride.
- Reactivity:
- Reacts with strong bases to form dichlorocarbene.
- Can be further chlorinated to form carbon tetrachloride.
- Reacts with hydrogen fluoride to produce difluorochloromethane.
Uses
- Medical Uses: Historically used as an anesthetic but is no longer recommended due to its hepatotoxicity.
- Industrial Uses: Used as a solvent for fats, oils, resins, and waxes; also used in organic synthesis.
- Other Uses: Formerly used in dry cleaning and as a refrigerant.
Safety Information
- Toxicity: Highly toxic; inhalation, ingestion, and skin contact can cause severe health effects, including liver and kidney damage.
- Health Hazards: Causes severe irritation to skin and eyes; inhalation may lead to central nervous system depression.
- First Aid:
- Skin Contact: Rinse thoroughly with soap and water.
- Eye Contact: Rinse with plenty of water for at least 15 minutes.
- Inhalation: Move to fresh air and seek medical attention.
- Ingestion: Do not induce vomiting; seek medical attention.
- Storage: Store in a cool, well-ventilated area, away from light and heat sources.
Environmental Information
- Hazardous Decomposition Products: Phosgene and hydrogen chloride.
- Ecotoxicity: Harmful to aquatic life.
Transport Information
- UN Number: UN1888.
- Hazard Class: 6.1 (Poisonous).
Regulatory Information
- Classification: Suspected human carcinogen (GHS Category 2).
- Occupational Exposure Limit: PC-TWA 20 mg/m³.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping