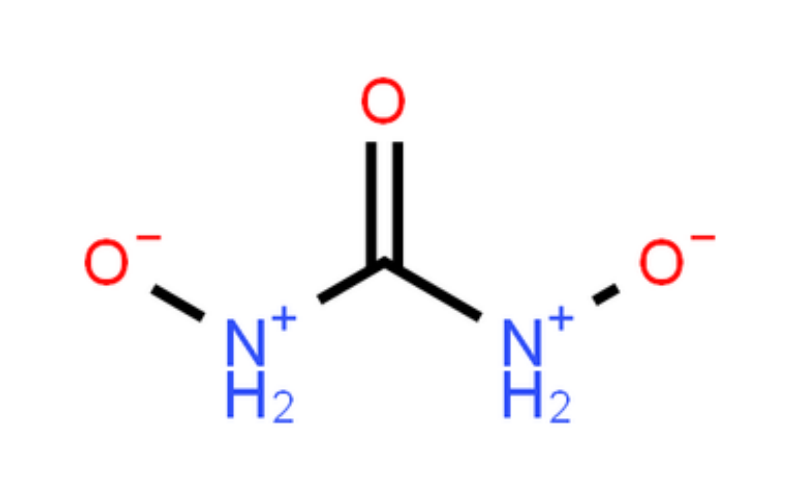
Physical Properties
- Appearance: White crystalline powder
- Melting Point: 90-93°C (lit.)
- Boiling Point: 175.5°C (rough estimate)
- Solubility: Soluble in water (500 g/L at 20°C)
- Stability: Unstable; oxidizer; severe explosion hazard on exposure to shock or heat
Chemical Properties
Urea Hydrogen Peroxide is a compound formed by the strong hydrogen bonding between urea and hydrogen peroxide. It combines the properties of both urea and hydrogen peroxide, making it a versatile oxidizing agent. It is highly reactive in the presence of acids, bases, or catalysts and can decompose to release oxygen.
Synthesis
Urea Hydrogen Peroxide is typically synthesized by reacting urea with hydrogen peroxide in an aqueous solution. The process involves forming a 1:1 complex between urea and hydrogen peroxide.
Applications
- Bleaching and Whitening: Widely used in hair care products, teeth whitening agents, and as a bleaching agent in neutral detergents.
- Disinfection: Acts as a safe and effective disinfectant in water treatment and surface cleaning.
- Organic Synthesis: Used as an oxidizing agent in various organic reactions, such as epoxidation of double bonds and oxidation of heteroatoms (N, S, P, etc.).
- Pharmaceuticals and Cosmetics: Used in products for its antibacterial and bleaching properties.
Safety Information
- Hazard Class: Oxidizer (O), Corrosive (C)
- Risk Phrases: R8 (Contact with combustible material may cause fire); R34 (Causes burns)
- Safety Phrases: S17 (Keep away from combustible material); S26 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice); S36/37/39 (Wear suitable protective clothing, gloves, and eye/face protection); S45 (In case of accident or if you feel unwell, seek medical advice immediately)
Storage and Handling
- Store in a cool, dry place, away from heat and sources of ignition.
- Keep in tightly closed containers and avoid contact with reducing agents and organic materials.
Our company specializes in hazardous chemicals, flammable and explosive chemicals, toxic chemicals (legal export), ultra-pure and high-purity reagents. Welcome to contact us.
Packing and shipping